Why corrosion happens
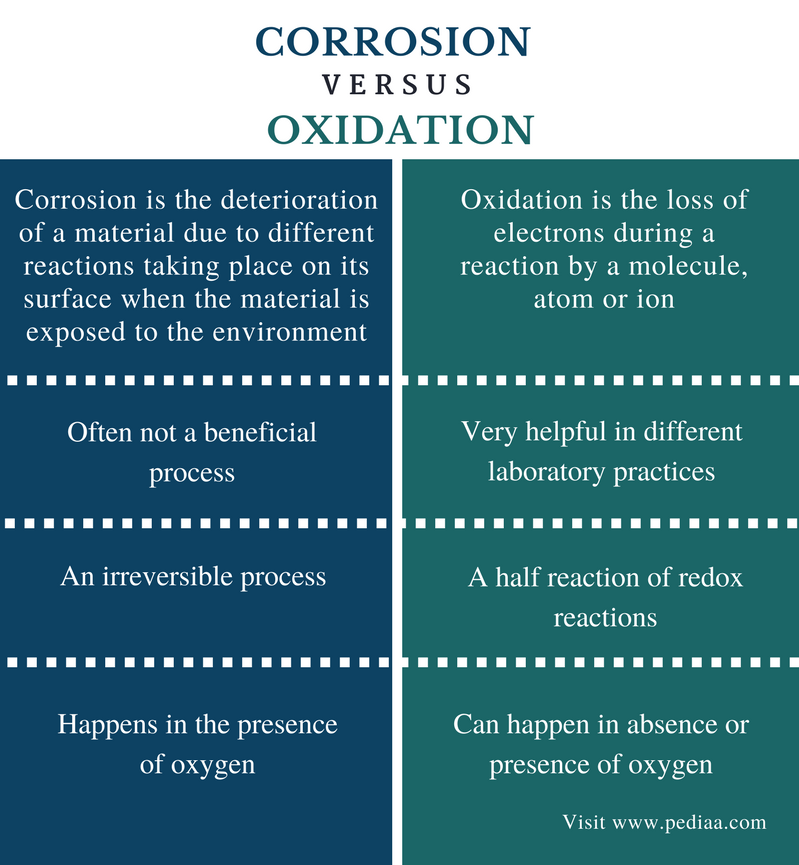
Why Corrosion Happens. In the most common use of the word this means. Most metals are easily oxidized. They tend to lose electrons to oxygen and other substances in the air or in water. Corrosion is the deterioration of a metal as a result of chemical reactions between it and the surrounding environment.
 How Does Pitting Corrosion Occur Localised Corrosion Electrochemical Corrosion Youtube From youtube.com
How Does Pitting Corrosion Occur Localised Corrosion Electrochemical Corrosion Youtube From youtube.com
Corrosion occurs when the metal begins to release this energy after oxidizing agents in the environment trigger a chemical reaction the most common oxidizing agent being oxygen. Most metals are easily oxidized. They tend to lose electrons to oxygen and other substances in the air or in water. It is the gradual destruction of materials usually a metal by chemical and or electrochemical reaction with their environment. In the most common use of the word this means. Corrosion is the deterioration of a metal as a result of chemical reactions between it and the surrounding environment.
They tend to lose electrons to oxygen and other substances in the air or in water.
Corrosion engineering is the field dedicated to controlling and preventing corrosion. Both the type of metal and the environmental conditions particularly gasses that are in contact with the metal determine the form and rate of deterioration. Corrosion engineering is the field dedicated to controlling and preventing corrosion. In the most common use of the word this means. It is the gradual destruction of materials usually a metal by chemical and or electrochemical reaction with their environment. Corrosion is the deterioration of a metal as a result of chemical reactions between it and the surrounding environment.
 Source: wileymetal.com
Source: wileymetal.com
Most metals are easily oxidized. They tend to lose electrons to oxygen and other substances in the air or in water. General corrosion occurs when most or all of the atoms on the same metal surface are oxidized damaging the entire surface. Corrosion is the deterioration of a metal as a result of chemical reactions between it and the surrounding environment. Corrosion engineering is the field dedicated to controlling and preventing corrosion.
 Source: thoughtco.com
Source: thoughtco.com
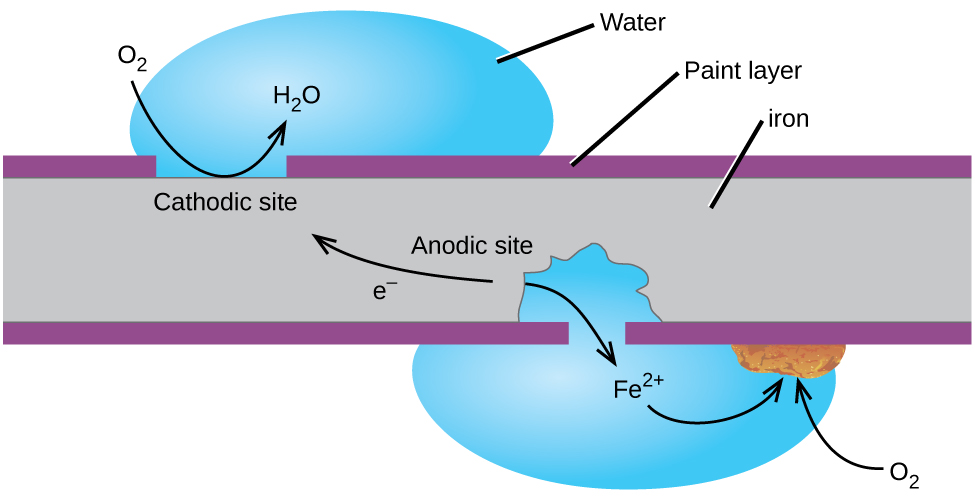
As oxygen is reduced gains electrons it forms an oxide with the metal. They tend to lose electrons to oxygen and other substances in the air or in water. General corrosion occurs when most or all of the atoms on the same metal surface are oxidized damaging the entire surface. Corrosion is the deterioration of a metal as a result of chemical reactions between it and the surrounding environment. As oxygen is reduced gains electrons it forms an oxide with the metal.
 Source: pediaa.com
Source: pediaa.com
Both the type of metal and the environmental conditions particularly gasses that are in contact with the metal determine the form and rate of deterioration. As oxygen is reduced gains electrons it forms an oxide with the metal. Corrosion engineering is the field dedicated to controlling and preventing corrosion. They tend to lose electrons to oxygen and other substances in the air or in water. Both the type of metal and the environmental conditions particularly gasses that are in contact with the metal determine the form and rate of deterioration.
 Source: slideplayer.com
Source: slideplayer.com
They tend to lose electrons to oxygen and other substances in the air or in water. Corrosion is a natural process that converts a refined metal into a more chemically stable form such as oxide hydroxide or sulfide. As oxygen is reduced gains electrons it forms an oxide with the metal. It is the gradual destruction of materials usually a metal by chemical and or electrochemical reaction with their environment. They tend to lose electrons to oxygen and other substances in the air or in water.
 Source: xapps.xyleminc.com
Source: xapps.xyleminc.com
It is the gradual destruction of materials usually a metal by chemical and or electrochemical reaction with their environment. Most metals are easily oxidized. Corrosion occurs when the metal begins to release this energy after oxidizing agents in the environment trigger a chemical reaction the most common oxidizing agent being oxygen. General corrosion occurs when most or all of the atoms on the same metal surface are oxidized damaging the entire surface. Both the type of metal and the environmental conditions particularly gasses that are in contact with the metal determine the form and rate of deterioration.
 Source: provenproductivity.com
Source: provenproductivity.com
Corrosion is a natural process that converts a refined metal into a more chemically stable form such as oxide hydroxide or sulfide. General corrosion occurs when most or all of the atoms on the same metal surface are oxidized damaging the entire surface. As oxygen is reduced gains electrons it forms an oxide with the metal. Both the type of metal and the environmental conditions particularly gasses that are in contact with the metal determine the form and rate of deterioration. Corrosion engineering is the field dedicated to controlling and preventing corrosion.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
As oxygen is reduced gains electrons it forms an oxide with the metal. Corrosion is a natural process that converts a refined metal into a more chemically stable form such as oxide hydroxide or sulfide. Both the type of metal and the environmental conditions particularly gasses that are in contact with the metal determine the form and rate of deterioration. Corrosion occurs when the metal begins to release this energy after oxidizing agents in the environment trigger a chemical reaction the most common oxidizing agent being oxygen. It is the gradual destruction of materials usually a metal by chemical and or electrochemical reaction with their environment.
 Source: ddcoatings.co.uk
Source: ddcoatings.co.uk
Corrosion is a natural process that converts a refined metal into a more chemically stable form such as oxide hydroxide or sulfide. Most metals are easily oxidized. General corrosion occurs when most or all of the atoms on the same metal surface are oxidized damaging the entire surface. In the most common use of the word this means. They tend to lose electrons to oxygen and other substances in the air or in water.
 Source: electrochem.org
Source: electrochem.org
Corrosion is the deterioration of a metal as a result of chemical reactions between it and the surrounding environment. Both the type of metal and the environmental conditions particularly gasses that are in contact with the metal determine the form and rate of deterioration. Corrosion engineering is the field dedicated to controlling and preventing corrosion. Most metals are easily oxidized. In the most common use of the word this means.
 Source: youtube.com
Source: youtube.com
As oxygen is reduced gains electrons it forms an oxide with the metal. They tend to lose electrons to oxygen and other substances in the air or in water. Corrosion is a natural process that converts a refined metal into a more chemically stable form such as oxide hydroxide or sulfide. It is the gradual destruction of materials usually a metal by chemical and or electrochemical reaction with their environment. As oxygen is reduced gains electrons it forms an oxide with the metal.
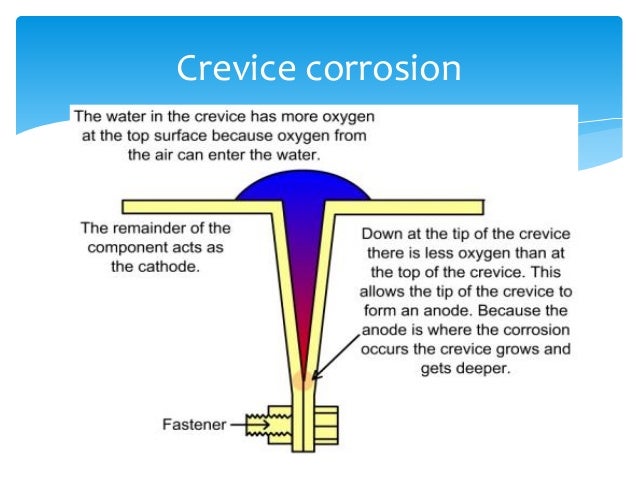 Source: slideshare.net
Source: slideshare.net
Corrosion occurs when the metal begins to release this energy after oxidizing agents in the environment trigger a chemical reaction the most common oxidizing agent being oxygen. Corrosion occurs when the metal begins to release this energy after oxidizing agents in the environment trigger a chemical reaction the most common oxidizing agent being oxygen. As oxygen is reduced gains electrons it forms an oxide with the metal. General corrosion occurs when most or all of the atoms on the same metal surface are oxidized damaging the entire surface. It is the gradual destruction of materials usually a metal by chemical and or electrochemical reaction with their environment.
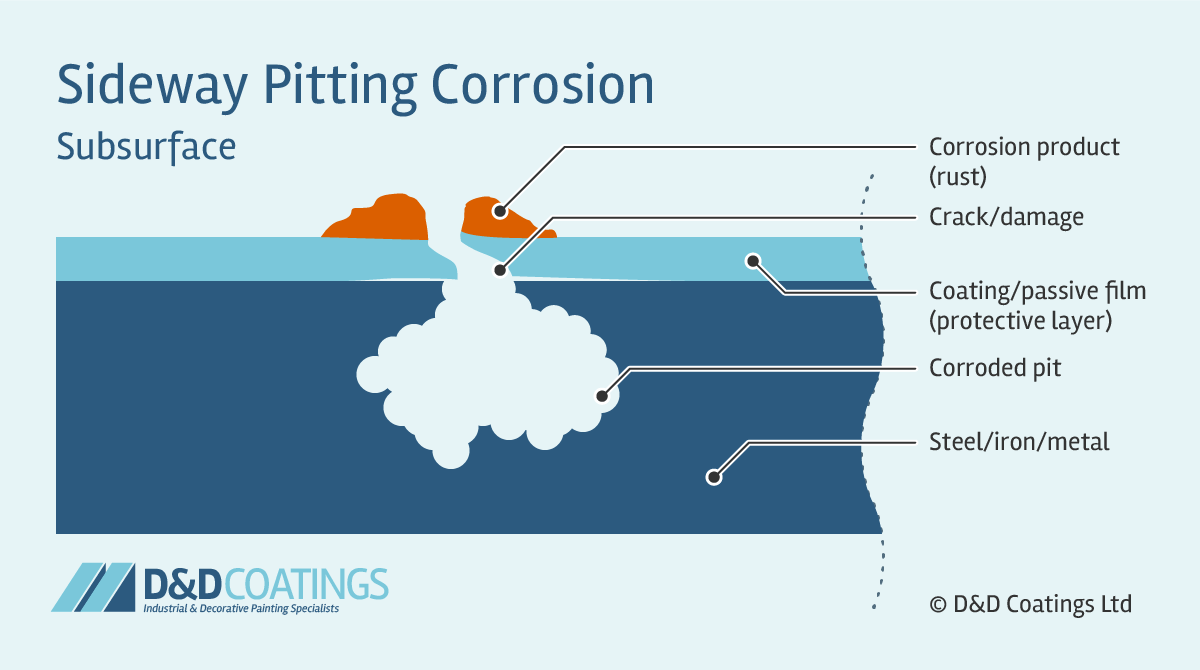 Source: ddcoatings.co.uk
Source: ddcoatings.co.uk
Both the type of metal and the environmental conditions particularly gasses that are in contact with the metal determine the form and rate of deterioration. Corrosion engineering is the field dedicated to controlling and preventing corrosion. It is the gradual destruction of materials usually a metal by chemical and or electrochemical reaction with their environment. They tend to lose electrons to oxygen and other substances in the air or in water. Corrosion is a natural process that converts a refined metal into a more chemically stable form such as oxide hydroxide or sulfide.
 Source: erps.com.au
Source: erps.com.au
As oxygen is reduced gains electrons it forms an oxide with the metal. Most metals are easily oxidized. Corrosion is a natural process that converts a refined metal into a more chemically stable form such as oxide hydroxide or sulfide. Corrosion is the deterioration of a metal as a result of chemical reactions between it and the surrounding environment. They tend to lose electrons to oxygen and other substances in the air or in water.
 Source: slideplayer.com
Source: slideplayer.com
They tend to lose electrons to oxygen and other substances in the air or in water. Corrosion is the deterioration of a metal as a result of chemical reactions between it and the surrounding environment. Corrosion is a natural process that converts a refined metal into a more chemically stable form such as oxide hydroxide or sulfide. As oxygen is reduced gains electrons it forms an oxide with the metal. They tend to lose electrons to oxygen and other substances in the air or in water.
 Source: slideshare.net
Source: slideshare.net
It is the gradual destruction of materials usually a metal by chemical and or electrochemical reaction with their environment. Most metals are easily oxidized. In the most common use of the word this means. It is the gradual destruction of materials usually a metal by chemical and or electrochemical reaction with their environment. General corrosion occurs when most or all of the atoms on the same metal surface are oxidized damaging the entire surface.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title why corrosion happens by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.