Why does baking soda react to vinegar
Why Does Baking Soda React To Vinegar. They react because one is a base while the other is an acid. Because carbon dioxide is heavier than air it displaces it. Carbonic acid and sodium acetate. The carbon dioxide released by the baking soda and vinegar reaction has other uses besides making a chemical volcano.
 Bonding Process From bakingsodaandvinegarbonding.weebly.com
Bonding Process From bakingsodaandvinegarbonding.weebly.com
This creates the bubbles and foam you see when you mix baking soda and vinegar. When vinegar and baking soda are first mixed together hydrogen ions in the vinegar react with the sodium and bicarbonate ions in the baking soda. Baking soda contains one sodium atom one hydrogen atom one oxygen atom and one carbon dioxide molecule. Baking soda is a basic compound called sodium bicarbonate. If the mixture is strong then the froth is released more and faster. The reaction causes the baking soda to transform into water and carbon dioxide.
It s really a double replacement reaction.
The acetic acid that s what makes vinegar sour reacts with sodium bicarbonate a compound that s in baking soda to form carbonic acid. The reaction between the two substances results in a violent form. When vinegar and baking soda are first mixed together hydrogen ions in the vinegar react with the sodium and bicarbonate ions in the baking soda. The reaction also results into bubbling because of the production of gaseous carbon dioxide. The acetic acid that s what makes vinegar sour reacts with sodium bicarbonate a compound that s in baking soda to form carbonic acid. Baking soda is a basic compound called sodium bicarbonate.
 Source: middleschoolchemistry.com
Source: middleschoolchemistry.com
This creates the bubbles and foam you see when you mix baking soda and vinegar. Baking soda contains one sodium atom one hydrogen atom one oxygen atom and one carbon dioxide molecule. If you add a little washing up liquid dish soap the foam becomes thick a little like lava. During the reaction when the baking soda is mixed with the vinegar the baking soda base takes a proton from the vinegar acid. Carbonic acid is unstable and it immediately falls apart into carbon dioxide and water it s a decomposition reaction.
 Source: stemmayhem.com
Source: stemmayhem.com
The baking soda generates an acidic reaction with the vinegar. The baking soda and vinegar reaction is actually two separate reactions. Baking soda is a basic compound called sodium bicarbonate. What actually happens is this. Baking soda contains one sodium atom one hydrogen atom one oxygen atom and one carbon dioxide molecule.
 Source: phys.org
Source: phys.org
Sodium bicarbonate commonly referred to as baking soda and acetic acid which is vinegar combine to create an acid base chemical reaction. The baking soda and vinegar reaction is actually two separate reactions. They react because one is a base while the other is an acid. When vinegar and baking soda are first mixed together hydrogen ions in the vinegar react with the sodium and bicarbonate ions in the baking soda. The result of this initial reaction is two new chemicals.
 Source: alamy.com
Source: alamy.com
When vinegar and baking soda are first mixed together hydrogen ions in the vinegar react with the sodium and bicarbonate ions in the baking soda. Baking soda contains one sodium atom one hydrogen atom one oxygen atom and one carbon dioxide molecule. Bases contain an oh or. The result of this initial reaction is two new chemicals. The baking soda and vinegar reaction is actually two separate reactions.
 Source: chemistry291.blogspot.com
Source: chemistry291.blogspot.com
Baking soda is a basic compound called sodium bicarbonate. Vinegar baking soda video. Because carbon dioxide is heavier than air it displaces it. Bases contain an oh or. The baking soda generates an acidic reaction with the vinegar.
 Source: bakingsodaandvinegarbonding.weebly.com
Source: bakingsodaandvinegarbonding.weebly.com
The acetic acid that s what makes vinegar sour reacts with sodium bicarbonate a compound that s in baking soda to form carbonic acid. The first reaction is the acid base reaction. The baking soda serves as the base and the vinegar is the acid. Bases contain an oh or. Baking soda is a basic compound called sodium bicarbonate.
 Source: welcomingwildlife.com
Source: welcomingwildlife.com
When vinegar and baking soda are first mixed together hydrogen ions in the vinegar react with the sodium and bicarbonate ions in the baking soda. If the mixture is strong then the froth is released more and faster. Baking soda and vinegar react to neutralise each other vinegar is an acid and baking soda an alkali releasing carbon dioxide which is the bubbles of gas you see. The first reaction is the acid base reaction. The baking soda generates an acidic reaction with the vinegar.
 Source: slideserve.com
Source: slideserve.com
If the mixture is strong then the froth is released more and faster. Baking soda contains one sodium atom one hydrogen atom one oxygen atom and one carbon dioxide molecule. The reaction between the two substances results in a violent form. If you add a little washing up liquid dish soap the foam becomes thick a little like lava. Sodium bicarbonate commonly referred to as baking soda and acetic acid which is vinegar combine to create an acid base chemical reaction.
 Source: m.youtube.com
Source: m.youtube.com
Carbonic acid is unstable and it immediately falls apart into carbon dioxide and water it s a decomposition reaction. The reaction causes the baking soda to transform into water and carbon dioxide. Bases contain an oh or. An acid base reaction occurs after mixing vinegar and baking soda according to the ucsb scienceline website. The reaction between the two substances results in a violent form.
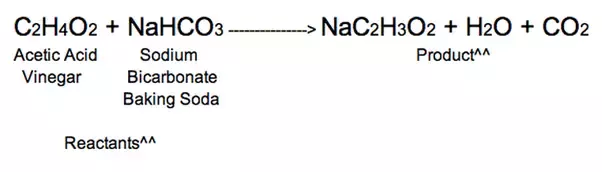 Source: quora.com
Source: quora.com
Vinegar is a diluted solution that contains acetic acid. The acetic acid that s what makes vinegar sour reacts with sodium bicarbonate a compound that s in baking soda to form carbonic acid. This starves a fire of the oxygen needed for combustion. During the reaction when the baking soda is mixed with the vinegar the baking soda base takes a proton from the vinegar acid. It s really a double replacement reaction.
 Source: alamy.com
Source: alamy.com
If the mixture is strong then the froth is released more and faster. Vinegar is a diluted solution that contains acetic acid. Baking soda and vinegar react chemically because one is a base and the other is an acid. The result of this initial reaction is two new chemicals. Bases contain an oh or.
 Source: chemistrycachet.com
Source: chemistrycachet.com
This starves a fire of the oxygen needed for combustion. Baking soda and vinegar react chemically because one is a base and the other is an acid. This starves a fire of the oxygen needed for combustion. Baking soda is a basic compound called sodium bicarbonate. The reaction causes the baking soda to transform into water and carbon dioxide.
 Source: youtube.com
Source: youtube.com
Acids and bases each have different parts of h2o or water. Carbonic acid is unstable and it immediately falls apart into carbon dioxide and water it s a decomposition reaction. Baking soda and vinegar react due to the exchange of atoms that occurs after combining them. This starves a fire of the oxygen needed for combustion. Acids and bases each have different parts of h2o or water.
 Source: funathomewithkids.com
Source: funathomewithkids.com
The result of this initial reaction is two new chemicals. The reaction causes the baking soda to transform into water and carbon dioxide. Baking soda is a basic compound called sodium bicarbonate. The carbon dioxide is a gas which is released during the reaction which gives it the bubbling effect and it expands which will blow up balloons as you have probably seen in some experiments and demonstrations. The result of this initial reaction is two new chemicals.
 Source: m.youtube.com
Source: m.youtube.com
The carbon dioxide released by the baking soda and vinegar reaction has other uses besides making a chemical volcano. The reaction between the two substances results in a violent form. The reaction also results into bubbling because of the production of gaseous carbon dioxide. Vinegar baking soda video. Carbonic acid is unstable and it immediately falls apart into carbon dioxide and water it s a decomposition reaction.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title why does baking soda react to vinegar by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.







