Why is calcium hydroxide called lime water
Why Is Calcium Hydroxide Called Lime Water. Calcium hydroxide traditionally called slaked lime is an inorganic compound with the chemical formula ca 2 it is a colorless crystal or white powder and is produced when quicklime calcium oxide is mixed or slaked with water it has many names including hydrated lime caustic lime builders lime slack lime cal and pickling lime calcium hydroxide is used in many applications including. Calcium hydroxide also called slaked lime ca oh is obtained by the action of water on calcium oxide. When co2 is passed through lime water insoluble precipitate caco3. Because of all these advantages explained in more detail below calcium oxide cao and calcium hydroxide ca oh 2 are used in drinking water purification plants.
 1 By Engr Dr Attaullah Shah 2 Lime Properties Chemistry Manufacturing And Uses Swedish College Of Engineering And Technology Ppt Download From slideplayer.com
1 By Engr Dr Attaullah Shah 2 Lime Properties Chemistry Manufacturing And Uses Swedish College Of Engineering And Technology Ppt Download From slideplayer.com
The suspension of calcium hydroxide particles in water is called milk of lime. Due to electrolyte dissociation this compound liberates oh ions. Answered june 7 2018. When mixed with water a small proportion of it dissolves forming a solution known as limewater the rest remaining as a suspension called milk of lime. When mixed with water a small proportion of it dissolves forming a solution known as limewater the rest remaining as a suspension called milk of lime. Lime water turns milky with carbon dioxide when passed over it due to the formation of insoluble caco3 calium carbonate.
A lot of heat evolved during the hydration.
Answered june 7 2018. Limewater is the common name for a dilute aqueous solution of calcium hydroxide calcium hydroxide ca oh 2 is sparsely soluble at room temperature in water 1 5 g l at 25 c. By chemical charectestics the quick lime has anhydrous property. Calcium hydroxide lime there are many chemicals available on the market today that are suitable for use as neutralization chemicals. Lime water turns milky with carbon dioxide when passed over it due to the formation of insoluble caco3 calium carbonate. The suspension of calcium hydroxide particles in water is called milk of lime.
 Source: sliderbase.com
Source: sliderbase.com
Because of all these advantages explained in more detail below calcium oxide cao and calcium hydroxide ca oh 2 are used in drinking water purification plants. When mixed with water a small proportion of it dissolves forming a solution known as limewater the rest remaining as a suspension called milk of lime. The process of adding of water is called hydration. Less than or fully saturated limewater is clear and colorless with a slight earthy smell and an astringent bitter taste. By chemical charectestics the quick lime has anhydrous property.
 Source: sodimate-inc.com
Source: sodimate-inc.com
The most commonly used chemicals are discussed in an article available here. Calcium hydroxide is formed as a result of. Because of all these advantages explained in more detail below calcium oxide cao and calcium hydroxide ca oh 2 are used in drinking water purification plants. When we pass excess co2 calcium bicarbonate can hco3 formed which is soluble in water so the solution turns transparent. A lot of heat evolved during the hydration.

Compounds calcium hydroxide also called slaked lime ca oh 2 is obtained by the action of water on calcium oxide. Limewater is the common name for a dilute aqueous solution of calcium hydroxide calcium hydroxide ca oh 2 is sparsely soluble at room temperature in water 1 5 g l at 25 c. The suspension of calcium hydroxide particles in water is called milk of lime. Calcium hydroxide lime there are many chemicals available on the market today that are suitable for use as neutralization chemicals. Calcium hydroxide traditionally called slaked lime is an inorganic compound with the chemical formula ca 2 it is a colorless crystal or white powder and is produced when quicklime calcium oxide is mixed or slaked with water it has many names including hydrated lime caustic lime builders lime slack lime cal and pickling lime calcium hydroxide is used in many applications including.
 Source: slideplayer.com
Source: slideplayer.com
The suspension of calcium hydroxide particles in water is called milk of lime. Compounds calcium hydroxide also called slaked lime ca oh 2 is obtained by the action of water on calcium oxide. When water is added by a controlled rate to the quick lime it turns in hydration form called calcium hydroxide which is also known as hydrated lime or slaked lime. When we pass excess co2 calcium bicarbonate can hco3 formed which is soluble in water so the solution turns transparent. Due to electrolyte dissociation this compound liberates oh ions.
 Source: slideshare.net
Source: slideshare.net
Because of all these advantages explained in more detail below calcium oxide cao and calcium hydroxide ca oh 2 are used in drinking water purification plants. Calcium hydroxide is formed as a result of. When mixed with water a small proportion of it dissolves forming a solution known as limewater the rest remaining as a suspension called milk of lime. Compounds calcium hydroxide also called slaked lime ca oh 2 is obtained by the action of water on calcium oxide. By chemical charectestics the quick lime has anhydrous property.
Source:
Answered june 7 2018. Calcium hydroxide is formed as a result of. When we pass excess co2 calcium bicarbonate can hco3 formed which is soluble in water so the solution turns transparent. Calcium hydroxide traditionally called slaked lime is an inorganic compound with the chemical formula ca 2 it is a colorless crystal or white powder and is produced when quicklime calcium oxide is mixed or slaked with water it has many names including hydrated lime caustic lime builders lime slack lime cal and pickling lime calcium hydroxide is used in many applications including. Calcium hydroxide also called slaked lime ca oh is obtained by the action of water on calcium oxide.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Because of all these advantages explained in more detail below calcium oxide cao and calcium hydroxide ca oh 2 are used in drinking water purification plants. The most commonly used chemicals are discussed in an article available here. When we pass excess co2 calcium bicarbonate can hco3 formed which is soluble in water so the solution turns transparent. When water is added by a controlled rate to the quick lime it turns in hydration form called calcium hydroxide which is also known as hydrated lime or slaked lime. When co2 is passed through lime water insoluble precipitate caco3.
 Source: issuu.com
Source: issuu.com
Lime water turns milky with carbon dioxide when passed over it due to the formation of insoluble caco3 calium carbonate. When mixed with water a small proportion of it dissolves forming a solution known as limewater the rest remaining as a suspension called milk of lime. The process of adding of water is called hydration. Limewater is the common name for a dilute aqueous solution of calcium hydroxide calcium hydroxide ca oh 2 is sparsely soluble at room temperature in water 1 5 g l at 25 c. Calcium hydroxide also called slaked lime ca oh is obtained by the action of water on calcium oxide.
 Source: skychem.en.made-in-china.com
Source: skychem.en.made-in-china.com
Compounds calcium hydroxide also called slaked lime ca oh 2 is obtained by the action of water on calcium oxide. A lot of heat evolved during the hydration. Because of all these advantages explained in more detail below calcium oxide cao and calcium hydroxide ca oh 2 are used in drinking water purification plants. Accordingly lime allows water to be softened purified have its cloudiness eliminated its acidity to be neutralized and its impurities to be eliminated etc. When mixed with water a small proportion of it dissolves forming a solution known as limewater the rest remaining as a suspension called milk of lime.
 Source: britannica.com
Source: britannica.com
The process of adding of water is called hydration. The solubility decreases with increasing temperature. The most commonly used chemicals are discussed in an article available here. Calcium hydroxide ca oh 2 calcium hydroxide ca oh 2 also commonly referred to as slaked lime or hydrated lime. When water is added by a controlled rate to the quick lime it turns in hydration form called calcium hydroxide which is also known as hydrated lime or slaked lime.
 Source: youtube.com
Source: youtube.com
Answered june 7 2018. The process of adding of water is called hydration. When mixed with water a small proportion of it dissolves forming a solution known as limewater the rest remaining as a suspension called milk of lime. Calcium hydroxide also known as slaked lime with the chemical formula ca oh 2 is a source of hydroxide ions when dissolved in aqueous solutions. Calcium hydroxide ca oh 2 calcium hydroxide ca oh 2 also commonly referred to as slaked lime or hydrated lime.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The suspension of calcium hydroxide particles in water is called milk of lime. Calcium hydroxide ca oh 2 calcium hydroxide ca oh 2 also commonly referred to as slaked lime or hydrated lime. Lime water turns milky with carbon dioxide when passed over it due to the formation of insoluble caco3 calium carbonate. When co2 is passed through lime water insoluble precipitate caco3. Accordingly lime allows water to be softened purified have its cloudiness eliminated its acidity to be neutralized and its impurities to be eliminated etc.
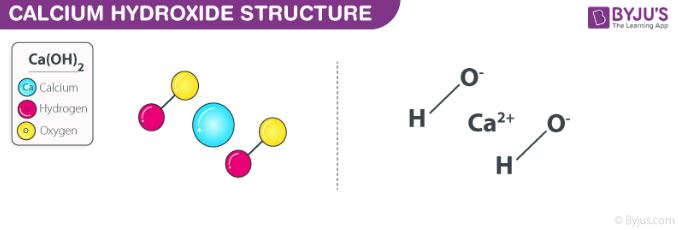 Source: byjus.com
Source: byjus.com
When co2 is passed through lime water insoluble precipitate caco3. Lime water turns milky with carbon dioxide when passed over it due to the formation of insoluble caco3 calium carbonate. Therefore this compound is a base. Calcium hydroxide ca oh 2 calcium hydroxide ca oh 2 also commonly referred to as slaked lime or hydrated lime. When we pass excess co2 calcium bicarbonate can hco3 formed which is soluble in water so the solution turns transparent.
 Source: mybenta.com
Source: mybenta.com
Calcium hydroxide lime there are many chemicals available on the market today that are suitable for use as neutralization chemicals. The solubility decreases with increasing temperature. When we pass excess co2 calcium bicarbonate can hco3 formed which is soluble in water so the solution turns transparent. The most commonly used chemicals are discussed in an article available here. When co2 is passed through lime water insoluble precipitate caco3.
 Source: mistralni.co.uk
Source: mistralni.co.uk
Because of all these advantages explained in more detail below calcium oxide cao and calcium hydroxide ca oh 2 are used in drinking water purification plants. When we pass excess co2 calcium bicarbonate can hco3 formed which is soluble in water so the solution turns transparent. Due to electrolyte dissociation this compound liberates oh ions. Calcium hydroxide traditionally called slaked lime is an inorganic compound with the chemical formula ca 2 it is a colorless crystal or white powder and is produced when quicklime calcium oxide is mixed or slaked with water it has many names including hydrated lime caustic lime builders lime slack lime cal and pickling lime calcium hydroxide is used in many applications including. When water is added by a controlled rate to the quick lime it turns in hydration form called calcium hydroxide which is also known as hydrated lime or slaked lime.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title why is calcium hydroxide called lime water by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.