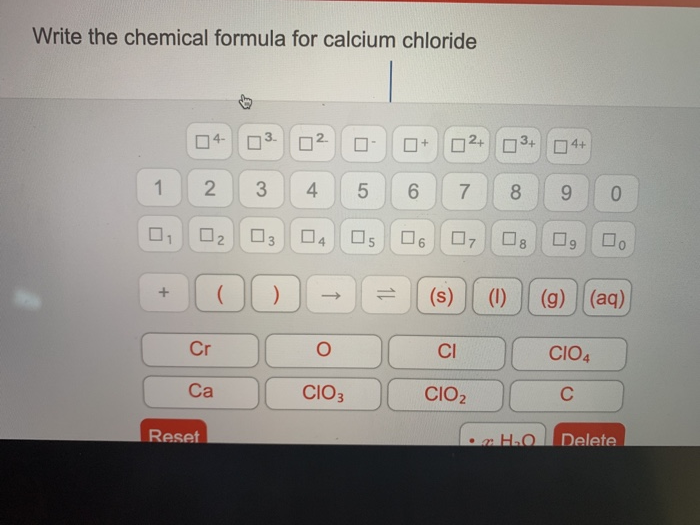
Write the formula for calcium chloride
Write The Formula For Calcium Chloride. Calcium is in group 2 it has a 2 charge. The chemical formula of calcium chloride is cacl2. The chemical formula for calcium chloride is cacl2 ca ionically bonded with 2 cl. Chlorine is a halogen 17 7b or viia and forms a 1 ion.
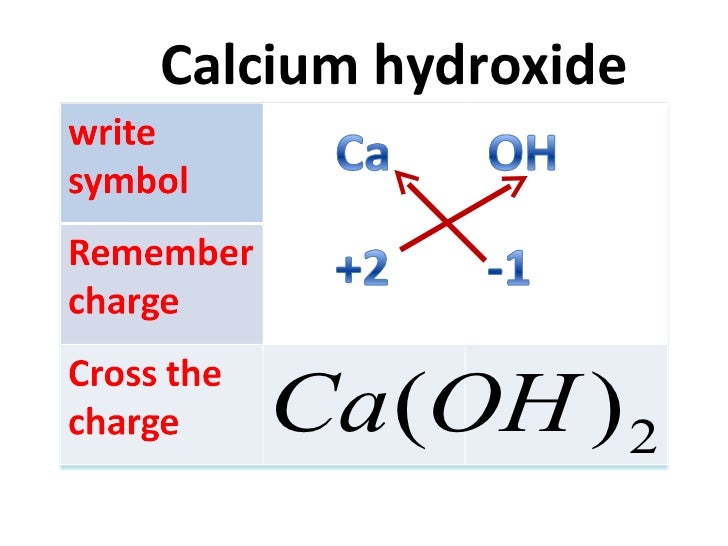 How To Write Chemical Formula From slideshare.net
How To Write Chemical Formula From slideshare.net
Calcium is in group 2 it has a 2 charge. It can be created by neutralising hydrochloric acid with calcium hydroxide. Writing the superscript of one ion as the subscript of the other gives the correct formula. Calcium chloride is commonly encountered as a hydrated solid with generic formula cacl 2 h 2 o x where x 0 1 2 4 and 6. Calcium chloride is hygroscopic deliquescent and dissolves in water exothermically. A video explanation of how to write the chemical formula for calcium chloride calcium chloride is an ionic compound and therefore we need to take into accoun.
The chemical formula for calcium chloride is cacl2 ca ionically bonded with 2 cl.
Therefore we need two chlorine ions to balance out the calcium ion charge as 2 2 0. Calcium chloride is an inorganic compound a salt with the chemical formula cacl 2 it is a white coloured crystalline solid at room temperature and it is highly soluble in water. Calcium chloride is commonly encountered as a hydrated solid with generic formula cacl 2 h 2 o x where x 0 1 2 4 and 6. Calcium is in group 2 it has a 2 charge. Chlorine is in group 7 it has a 1 charge. Therefore we need two chlorine ions to balance out the calcium ion charge as 2 2 0.
 Source: youtube.com
Source: youtube.com
Writing the superscript of one ion as the subscript of the other gives the correct formula. Cacl calciums symbol is ca and chlorines symbol is cl. It can be created by neutralising hydrochloric acid with calcium hydroxide. Therefore we need two chlorine ions to balance out the calcium ion charge as 2 2 0. The compound would be ionic formed from elements on opposite sides of the periodic table.
 Source: slideshare.net
Source: slideshare.net
Calcium is an alkaline earth metal 2 2a or iia which means it forms a 2 ion. The compound would be ionic formed from elements on opposite sides of the periodic table. A video explanation of how to write the chemical formula for calcium chloride calcium chloride is an ionic compound and therefore we need to take into accoun. The chemical formula for calcium chloride is cacl2 ca ionically bonded with 2 cl. As the overall charge is 0 neutral the charges must balance.
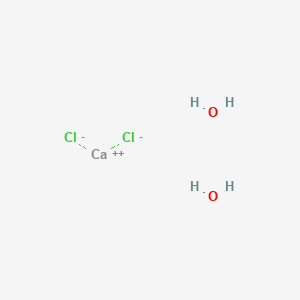
Calcium is an alkaline earth metal 2 2a or iia which means it forms a 2 ion. It can be created by neutralising hydrochloric acid with calcium hydroxide. As the overall charge is 0 neutral the charges must balance. Writing the superscript of one ion as the subscript of the other gives the correct formula. Chlorine is a halogen 17 7b or viia and forms a 1 ion.
 Source: youtube.com
Source: youtube.com
Chlorine is in group 7 it has a 1 charge. A video explanation of how to write the chemical formula for calcium chloride calcium chloride is an ionic compound and therefore we need to take into accoun. It can be created by neutralising hydrochloric acid with calcium hydroxide. Chlorine is a halogen 17 7b or viia and forms a 1 ion. The compound would be ionic formed from elements on opposite sides of the periodic table.
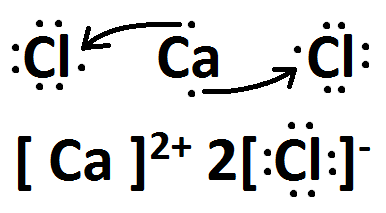 Source: socratic.org
Source: socratic.org
Calcium chloride is an inorganic compound a salt with the chemical formula cacl 2 it is a white coloured crystalline solid at room temperature and it is highly soluble in water. Calcium chloride is an inorganic compound a salt with the chemical formula cacl 2 it is a white coloured crystalline solid at room temperature and it is highly soluble in water. Chlorine is a halogen 17 7b or viia and forms a 1 ion. It is widely used as brine for refrigeration plants to replenish calcium levels as an antidote for magnesium poisoning and as an acid producing diuretic. Writing the superscript of one ion as the subscript of the other gives the correct formula.
 Source: alchetron.com
Source: alchetron.com
Therefore we need two chlorine ions to balance out the calcium ion charge as 2 2 0. Calcium is in group 2 it has a 2 charge. The chemical formula for calcium chloride is cacl2 ca ionically bonded with 2 cl. Therefore we need two chlorine ions to balance out the calcium ion charge as 2 2 0. As the overall charge is 0 neutral the charges must balance.
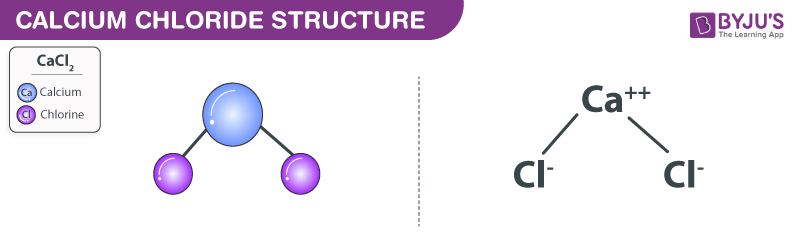 Source: byjus.com
Source: byjus.com
The compound would be ionic formed from elements on opposite sides of the periodic table. As the overall charge is 0 neutral the charges must balance. Writing the superscript of one ion as the subscript of the other gives the correct formula. Calcium is an alkaline earth metal 2 2a or iia which means it forms a 2 ion. The compound would be ionic formed from elements on opposite sides of the periodic table.
 Source: slideplayer.com
Source: slideplayer.com
The chemical formula of calcium chloride is cacl2. Writing the superscript of one ion as the subscript of the other gives the correct formula. Calcium is an alkaline earth metal 2 2a or iia which means it forms a 2 ion. Calcium chloride is commonly encountered as a hydrated solid with generic formula cacl 2 h 2 o x where x 0 1 2 4 and 6. Calcium chloride is an inorganic compound a salt with the chemical formula cacl 2 it is a white coloured crystalline solid at room temperature and it is highly soluble in water.
 Source: slideplayer.com
Source: slideplayer.com
A video explanation of how to write the chemical formula for calcium chloride calcium chloride is an ionic compound and therefore we need to take into accoun. A video explanation of how to write the chemical formula for calcium chloride calcium chloride is an ionic compound and therefore we need to take into accoun. Chlorine is a halogen 17 7b or viia and forms a 1 ion. The chemical formula for calcium chloride is cacl2 ca ionically bonded with 2 cl. It is widely used as brine for refrigeration plants to replenish calcium levels as an antidote for magnesium poisoning and as an acid producing diuretic.
 Source: chegg.com
Source: chegg.com
Calcium chloride is an inorganic compound a salt with the chemical formula cacl 2 it is a white coloured crystalline solid at room temperature and it is highly soluble in water. It is widely used as brine for refrigeration plants to replenish calcium levels as an antidote for magnesium poisoning and as an acid producing diuretic. The compound would be ionic formed from elements on opposite sides of the periodic table. Chlorine is in group 7 it has a 1 charge. Therefore we need two chlorine ions to balance out the calcium ion charge as 2 2 0.
 Source: youtube.com
Source: youtube.com
It can be created by neutralising hydrochloric acid with calcium hydroxide. Chlorine is in group 7 it has a 1 charge. Calcium chloride is hygroscopic deliquescent and dissolves in water exothermically. Calcium is in group 2 it has a 2 charge. Therefore we need two chlorine ions to balance out the calcium ion charge as 2 2 0.
 Source: chegg.com
Source: chegg.com
Calcium chloride is commonly encountered as a hydrated solid with generic formula cacl 2 h 2 o x where x 0 1 2 4 and 6. Calcium chloride is hygroscopic deliquescent and dissolves in water exothermically. It can be created by neutralising hydrochloric acid with calcium hydroxide. The chemical formula for calcium chloride is cacl2 ca ionically bonded with 2 cl. The chemical formula of calcium chloride is cacl2.

The chemical formula for calcium chloride is cacl2 ca ionically bonded with 2 cl. The compound would be ionic formed from elements on opposite sides of the periodic table. The chemical formula of calcium chloride is cacl2. Chlorine is in group 7 it has a 1 charge. Cacl calciums symbol is ca and chlorines symbol is cl.
Source: chemspider.com
It is widely used as brine for refrigeration plants to replenish calcium levels as an antidote for magnesium poisoning and as an acid producing diuretic. It is widely used as brine for refrigeration plants to replenish calcium levels as an antidote for magnesium poisoning and as an acid producing diuretic. Calcium chloride is commonly encountered as a hydrated solid with generic formula cacl 2 h 2 o x where x 0 1 2 4 and 6. The compound would be ionic formed from elements on opposite sides of the periodic table. As the overall charge is 0 neutral the charges must balance.
 Source: youtube.com
Source: youtube.com
Therefore we need two chlorine ions to balance out the calcium ion charge as 2 2 0. A video explanation of how to write the chemical formula for calcium chloride calcium chloride is an ionic compound and therefore we need to take into accoun. Cacl calciums symbol is ca and chlorines symbol is cl. The chemical formula for calcium chloride is cacl2 ca ionically bonded with 2 cl. Calcium chloride is an inorganic compound a salt with the chemical formula cacl 2 it is a white coloured crystalline solid at room temperature and it is highly soluble in water.
If you find this site helpful, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title write the formula for calcium chloride by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.







